
Evaluating Potential
Treatment Options
A Clinical Study for Adult Patients with Moderate-to-Severe
Thyroid Eye Disease (TED)
This information is intended for U.S. residents only.
Further information can be found in the study listings at
www.clinicaltrials.gov/study/NCT06307613 and www.clinicaltrials.gov/study/NCT06307626

Evaluating Potential
Treatment Options
A Clinical Study for Adult Patients with Moderate-to-Severe
Thyroid Eye Disease (TED)
This information is intended for U.S. residents only.
Further information can be found in the study listings at
www.clinicaltrials.gov/study/NCT06307613 and www.clinicaltrials.gov/study/NCT06307626

If you or someone you know is considering participating in a clinical study that is evaluating potential treatment options for managing Thyroid Eye Disease (TED), the UplighTED Study may be an option. The study is evaluating an investigational study drug, administered once a week, for its efficacy, safety, and tolerability in TED.


Thyroid Eye Disease (TED)
- Thyroid Eye Disease (TED) is sometimes called Graves’ Eye Disease.
- TED is an autoimmune disorder that affects the muscles and tissue around the eyes.
- In the active phase, swelling and bulging can cause pain and may affect vision.
- In the inactive phase, some symptoms may improve; however, eye bulging can persist.
- There is an unmet need in the management of the symptoms of TED.
Study Design
The UplighTED Study was designed in part from patients with TED.
Their input helped researchers better understand what it is like to live with TED.
A panel of patients were gathered where they provided feedback to researchers which helped to inform the study design. They shared with researchers symptoms they thought should be evaluated in the study to determine efficacy.
About the Study
- The study will evaluate the safety and efficacy of an investigational study drug compared to placebo (a substance that resembles the study drug but has no active ingredients) in adults with TED.
- Two out of three participants will receive the investigational study drug.
- One out of three participants will receive the placebo.
- Both the study drug and placebo are provided in prefilled syringes and administered via subcutaneous (under the skin) injection.
- Participants may choose to self-inject or have a caregiver perform the weekly injections.
- Participants may request a home nurse to assist with drug administration at home.
- The study may last up to two years.


Receiving Your Assigned Study Drug
- Study participants who are considered responders (i.e., show a reduction in the bulging of the eye at 24 weeks of treatment) will stop the investigational drug or placebo and be monitored for safety for 52 weeks.
- Participants who are considered non-responders (i.e., do not show a reduction in the bulging of the eye after the first 24 weeks of treatment) or those who have worsening during the follow up, may be eligible to enter the open label study where they will receive the investigational drug and be evaluated for safety and response to treatment.
Is This Study Right for You?
The participation criteria are below. Print this out and share with your specialist to decide together if the study is appropriate for you at this time.
- Be at least 18 years of age
- Be capable of providing signed informed consent and complying with study requirements
- Have received a diagnosis of active, moderate-to-severe TED associated with autoimmune thyroid disease (Graves’ disease or Hashimoto’s thyroiditis)
- Have ONSET of active symptoms such as redness, swelling, or pain of the eye or around the eye within the last 12 months, or recent worsening of the eye bulge
- Have hyperthyroidism or thyroid disease that is considered under control
*Study team members will determine if any of these apply to you
- You are currently participating in any clinical study with an investigational medication or intervention
- You have optic neuropathy, as defined by a decrease in vision or a new visual field defect, or another defect within the past six months prior to screening
- You stopped corticosteroids (also referred to as steroids), immunosuppressants (medicines that suppress your immune system) or monoclonal antibodies less than four weeks prior to screening
- You are currently taking other medications such as corticosteroids, immunosuppressants or monoclonal antibodies which you cannot stop
- You have received radioactive treatment for Graves’ disease within the past 6 months
- You’ve had previous orbital irradiation or surgery for TED or immediate planned eye surgery
- You have other autoimmune conditions or eye conditions which might interfere with the assessments of the study drug
*Study team members will determine if any of these apply to you
Download and Print the Study Fact
Sheet to Share with Your Specialist
MED-US-ESC-2400117
A Closer Look at Study Participation
The following is an overview on what is involved in participating in the UplighTED Study.
Screening Period
- Participants who decide to be screened, will be asked to sign an informed consent form (ICF). An ICF describes the study and any potential risks or benefits of participation. You can change your mind about participating at any time.
- Medical experts in TED will confirm whether or not you meet the criteria eligibility for this study.
- If you qualify for the study, and you decide to participate you will be randomly assigned to receive either the investigational study drug or a placebo which are both given through an injection by a prefilled syringe.
- This study is masked which means that neither the participant nor the study physician will know who is receiving the investigational study drug or the placebo.

Overview of Treatment
- The treatment (either the investigational study drug or placebo) will be administered with a prefilled syringe subcutaneously (under the skin).
Weekly Injections of the Investigational Study Drug
- These injections will be administered once weekly during the treatment period of the study.
- Injections may be done on study visit days or administered at home by yourself, a caregiver, or home nurse throughout the treatment period of the study.

Follow-up Period
- Study participants who are considered responders (show a reduction in the bulging of the eye at 24 weeks of treatment) will stop the investigational drug or placebo and be monitored for safety for 52 weeks.
- Participants who are considered non-responders (do not show a reduction in the bulging of the eye after the first 24 weeks of treatment) or those who have worsening during the follow up, may be eligible to enter the open label study where they will receive the investigational drug and be evaluated for safety and response to treatment.

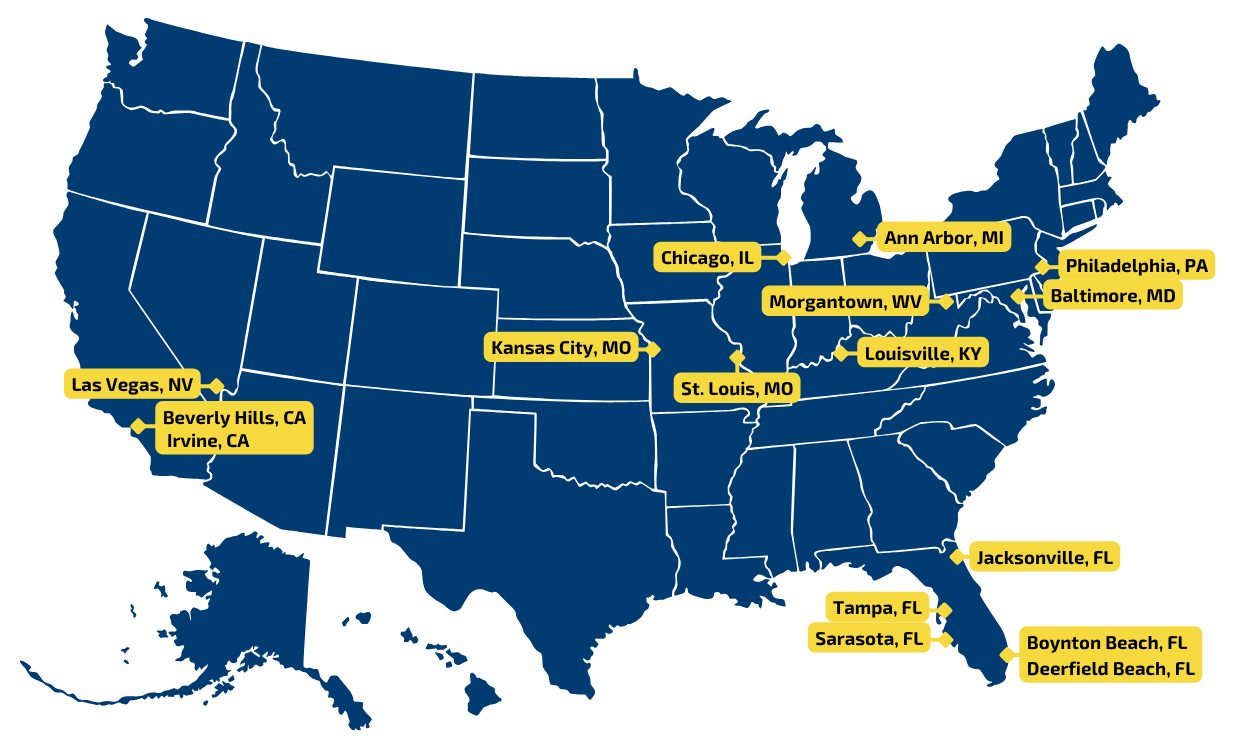
Locations
FAQs
Thyroid Eye Disease is an autoimmune disease that is diagnosed by examination and through blood tests. Some people are diagnosed by an endocrinologist with thyroid conditions like Graves’ Disease or Hashimoto’s Disease but also have TED (or Graves’ Eye Disease). Others experience the eye symptoms like proptosis (bulging eyes) and are diagnosed by an ophthalmologist. If you are diagnosed with TED, you may be referred by your physician to an ophthalmologist who specializes in TED.
When the participant experiences symptoms of TED, such as redness, worsening of eye bulging, pain, and swelling of the tissue around the eyes, it is considered ‘active’.
People choose to participate in a clinical study to personally contribute to the development of new treatments that can benefit people like yourself with TED.
Should you qualify to take part in the study and join it, you will receive (at no cost):
• Study drug
• Study-related medical care
- Learn if you may be eligible to participate by taking our brief online questionnaire. These health-related questions will help determine if you may be eligible to join the UplighTED Study.
- Have a phone call with a study representative to collect a few additional pieces of information related to your current TED treatment.
- If you meet the eligibility criteria, your information will be sent to a local study location where a member of the study team will contact you to answer any study-related questions you may have and to schedule an appointment for your in-person screening visit at the study location.
- Attend your in-person screening visit at the study location to do a final determination if you fully meet the requirements to participate, to better understand your responsibilities as a study participant and to decide if you want to participate. This visit is a good time to ask any questions you may have before you decide whether you want to participate in the UplighTED Study.
Participating in a clinical study is voluntary, allowing individuals control over their decision to join. Participants are able to withdraw from the study at any time and for any reason without any penalty. However, participants should recognize the responsibilities involved and consider whether they can meet the requirements before joining a study. Study representatives at the sites aim to provide support by ensuring your questions are answered and expectations are clear.
Participants will not be charged for study related visits or for the medications given as part of the study. Travel to study sites for study related visits may be reimbursed.
Sources:
- https://www.nei.nih.gov/learn-about-eye-health/eye-conditions-and-diseases/graves-eye-disease
- Szelog J, et al. Mo Med. 2022 Jul-Aug; 119(4): 343–350.
- https://clinicaltrials.gov/study/NCT06307613
- Dolman PJ. “Grading Severity and Activity in Thyroid Eye Disease.” Ophthal Plast Reconstr Surg 2018;34:S34–S40.
https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
