This website is intended for U.S. healthcare professionals only.

A Phase 3 Study to Evaluate the Efficacy, Safety and Tolerability of Efgartigimod SC Injection for Adults with Thyroid Eye Disease (TED)1
The investigational study drug, efgartigimod alfa with recombinant human hyaluronidase (PH20) SC, is not approved by any regulatory agency for the treatment of adult patients with thyroid eye disease (TED) as safety and efficacy have not been established.
About the Study
To evaluate efgartigimod PH20 SC given by prefilled syringe in adults with active, moderate-to-severe TED, compared with placebo PH20 SC.
Eligibility
Understand the inclusion/exclusion criteria of the study to determine if one of your patients may be eligible to participate.
Participation
After the screening period, participants will be randomized to 1 of 2 parallel arms to receive either investigational study drug or placebo (2:1 ratio investigational study drug:placebo).
Study Goal
To evaluate the efficacy, safety and tolerability of efgartigimod PH20 SC, administered via prefilled syringe, in adults with active, moderate-to-severe TED, compared with placebo PH20 SC.
Two phase 3, randomized, double-masked, placebo-controlled, multi-center studies are each enrolling approximately 108 adult participants with moderate-to-severe thyroid eye disease.

About Efgartigimod PH20 SC
Efgartigimod is a human antibody fragment that is modified to bind to a protein called FcRN. By binding to FcRN, efgartigimod prevents the binding of endogenous IgG to FcrN, leading to a reduction in IgGs including circulating disease-causing autoantibodies.2
The purpose of this study is to investigate efgartigimod’s efficacy, safety and tolerability in TED.
Efgartigimod alfa Proposed Mechanism of Action
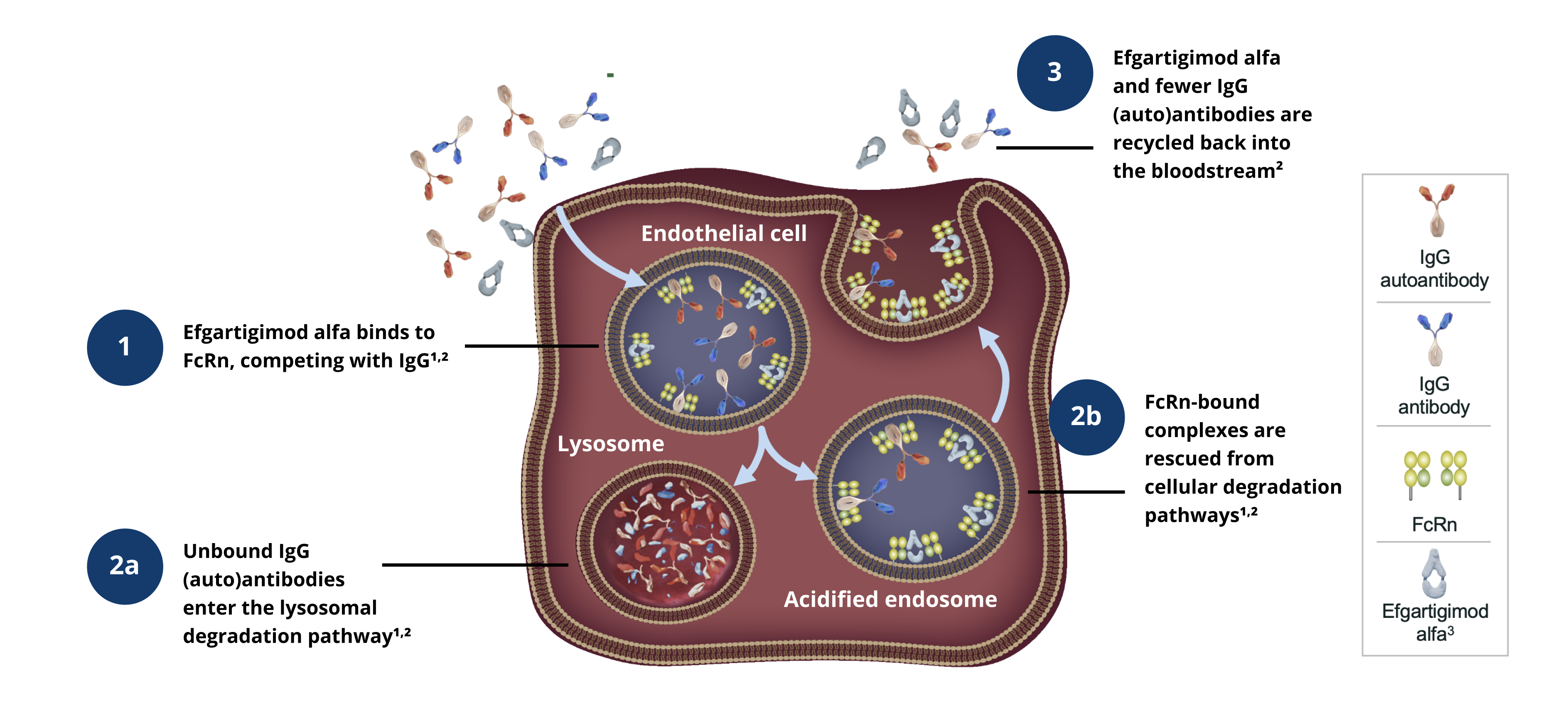
The investigational study drug, efgartigimod alfa with recombinant human hyaluronidase (PH20) SC, is not approved by any regulatory agency for the treatment of adult patients with thyroid eye disease (TED) as safety and efficacy have not been established.
1. Vaccaro C, et al. Nat Biotechnol. 2005;23(10):1283-1288. 2. Ulrichts P, et al. J Clin Invest. 2018;128(10):4372-4386. 3. Wolfe G, et al. J Neurol Sci. 2021;430:118074. The investigational study drug, efgartigimod alfa with recombinant human.
The investigational study drug, efgartigimod alfa with recombinant human hyaluronidase (PH20) SC, is not approved by any regulatory agency for the treatment of adult patients with thyroid eye disease (TED) as safety and efficacy have not been established.
Study Eligibility
Must meet all of the following criteria:
- Age 18 years or older
- Capable of providing signed informed consent
- Agrees to use contraceptive measures consistent with local regulations
- The participant has a physician’s diagnosis of active, moderate-to-severe thyroid eye disease (TED) associated with autoimmune thyroid conditions (Graves’ disease or Hashimoto’s thyroiditis)
- The participant has onset of active TED symptoms within 12 months before screening (Note: Active TED, defined as CAS≥4)
- The participant must have normal thyroid function with the baseline disease under control or have mild hypo or hyperthyroidism at screening
Patients may not be eligible to participate if the following applies:
- Optic neuropathy, defined as new visual field defect (blind spot), relative afferent pupillary defect (pupils respond differently to light), or color defect secondary to optic nerve involvement within the 6 months before screening
- Corneal decompensation unresponsive to medical management
- Previous orbital irradiation or surgery for TED
- Requires immediate eye surgery, or corrective surgery/irradiation therapy is planned during the study
- Use of certain medications before screening such as corticosteroids, immunosuppressants or monoclonal antibodies which cannot be stopped
- Known autoimmune disease or any medical condition that would interfere with an accurate assessment of clinical symptoms of TED or puts the participant at undue risk
- History of malignancy, cancer, unless considered cured by adequate treatment with no evidence of recurrence for ≥ 3 years before investigational drug administration
- Clinically significant active infection that is not sufficiently resolved in the investigator’s opinion or positive serum test at screening for active infection with any of the following: Hepatitis B virus (HBV), Hepatitis C virus (HCV), HIV
Further information can be found in the study listings at www.clinicaltrials.gov/study/NCT06307613 and www.clinicaltrials.gov/study/NCT06307626
Study Participation
Study Design
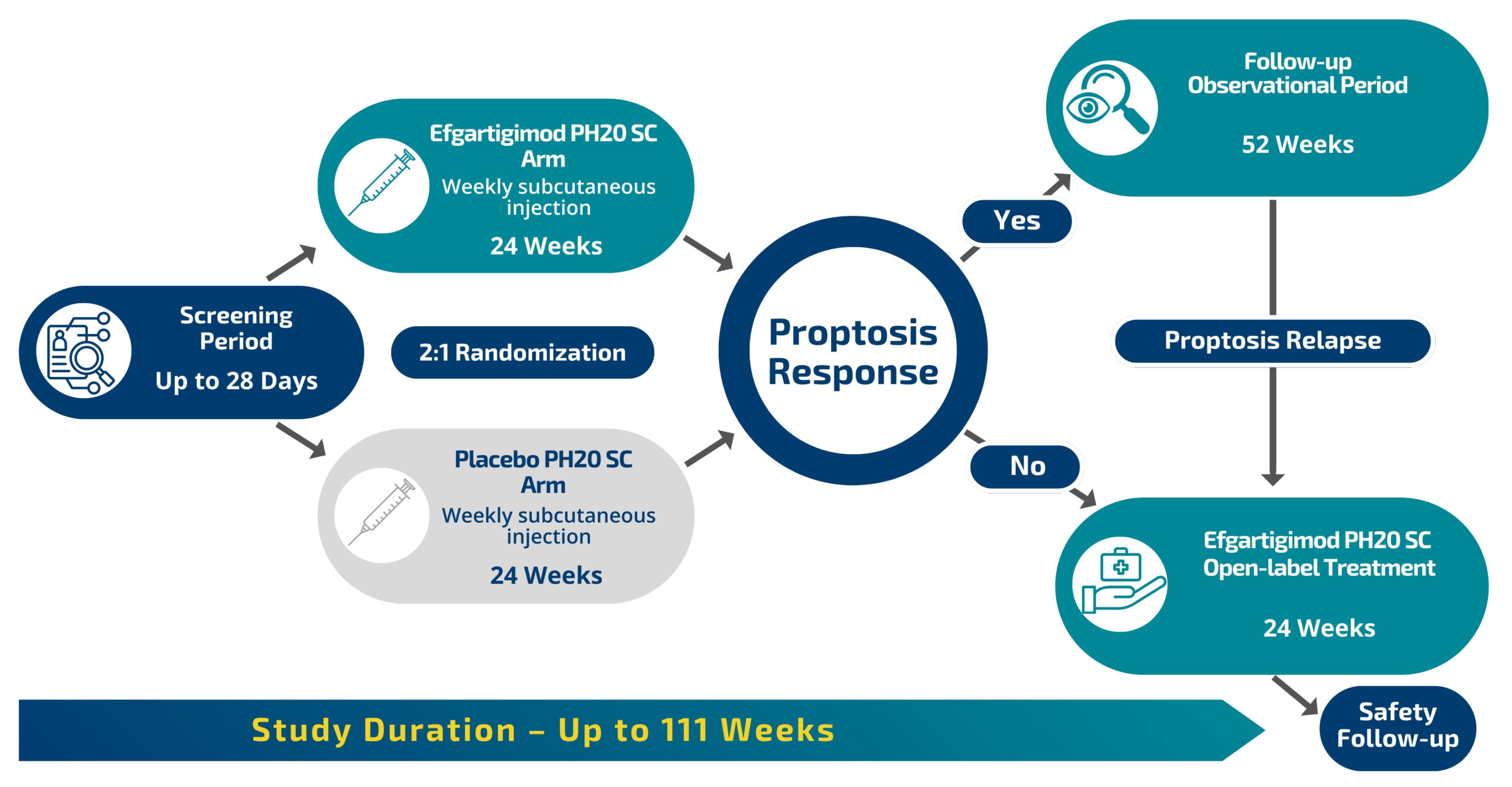
2:1 Chance of Receiving Investigational Study Drug vs Placebo
The study lasts 59-111 weeks. The initial treatment period, during which participants will receive weekly blinded study drug (investigational study drug or placebo), lasts 24 weeks, followed by a 52-week observational period. Individuals who don’t respond or who relapse (including placebo recipients) can be treated in an open label treatment period of 24 weeks at any time during follow-up. The study drug is dosed weekly and can be self-administered by prefilled syringe after training by study staff. Participants can also opt to have a caregiver or nurse to administer the study drug at home without the need to travel to the site outside of regular study visits.
The investigational study drug, efgartigimod alfa with recombinant human hyaluronidase (PH20) SC, is not approved by any regulatory agency for the treatment of adult patients with thyroid eye disease (TED) as safety and efficacy have not been established.
Interested in Referring Your Patients
with Moderate-to-Severe TED?
Contact Us to Refer a Patient
References:
- Clinicaltrials.gov ID: NCT06307613 and NCT06307626.
- Ulrichts et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans J Clin Invest. 2018; 128(10):4372-4386.
